Testing is designed to tell us things we want to know about individual cells and batteries.
Some typical questions are:
- Is it fully charged ?
- How much charge is left in the battery ?
- Does it meet the manufacturer's specification ?
- Has there been any deterioration in performance since it was new ?
- How long will it last ?
- Do the safety devices all work ?
- Does it generate interference or electrical noise ?
- Is it affected by interference or electrical noise ?
The answers are not always straightforward.
Indirect Measurements
Although all of the cell parameters the design engineer may wish to measure can be quantified by direct measurement, this is not always convenient or possible . For example the amount of charge left in the battery, the State of Charge (SOC) can be determined by fully discharging the battery and measuring the energy output. This takes time, it wastes energy, each test cycle shortens the battery life and it may not be practical if the battery is in use. It would also be pointless for a primary cell. For more detailed information of how this is done see the
State of Charge page.
Similarly, the remaining life of a secondary cell can be determined by continuously cycling it until it fails, but there's no point in knowing the cell life expectation if you have to destroy it to find out. This is known as the State of Health (SOH) of the battery.
What is needed are simple tests or measurements which can be used as an approximation to, or indirect measure of, the desired parameter. For more information see the
State of Health page
The Cell Design Process Testing
Test Conditions
In all of the following tests, and testing in general, the test conditions must be specified so that repeatable results can be obtained, and meaningful comparisons can be made. This includes factors such as method, temperature, DOD, load and duty cycle. For instance the cell capacity and cycle life, two key performance indicators could vary by 50% or more depending on the temperature and the discharge rate at which the tests were carried out. See also cell
Performance Characteristics.
Battery specifications should always include the test conditions to avoid ambiguity.
Qualification Testing
Qualification testing is designed to determine whether a cell or battery is fit for the purpose for which it was intended before it is approved for use in the product. This is particularly important if the cell is to be used in a "mission critical" application. These are comprehensive tests carried out initially on a small number of cells including testing some of them to destruction if necessary. As a second stage, qualification also includes testing finished battery packs before the product is approved for release to the customer. The tests are usually carried out to verify that the cells meet the manufacturer's specification but they could also be used to test the cells to arbitrary limits set by the applications engineer to determine how long the cells survive under adverse conditions or unusual loads, to determine failure modes or safety factors.
The battery packs should also be tested with the charger recommended for the application to ensure compatibility. In particular the potential user patterns must be evaluated to ensure that the batteries do not become inadvertently overcharged. See also the section on
Chargers.
Shake and Bake
- Mechanical Testing
Typical tests are included in the safety standards below. They include simple tests for dimensional accuracy to dynamic testing to verify that the product can survive any static and dynamic mechanical stresses to which it may be subject.
- Environmental Testing
Typical tests are included in the safety standards below. They are designed to exercise the product through all the environmental conditions likely to be encountered by the product during its lifetime.
Abuse Testing
The purpose of abuse testing is to verify that the battery is not a danger to the user or to itself either by accidental or deliberate abuse under any conceivable conditions of use. Designing foolproof batteries is ever more difficult because as we know, fools are so ingenious.
Abuse testing (always interesting to witness) is usually specified as part of the Safety Testing (below). Recent accidents with Lithium cells have highlighted the potential dangers and stricter battery design rules and a wider range of tests are being applied as well as stricter
Transport Regulationsfor shipping the products.
Consumer products normally have to comply with national or international
Safety Standards required by the safety organisations of the countries in which the products are sold. Examples are UL, ANSI, CSA and IEC standards.
Typical contents
Safety Tests
|
|---|
Casing
- Strength, rigidity and flammability
- Mould stress (Temperature)
- Venting
- Insulation
- Electrolyte not under pressure
- No leakage
- No explosion or fire risk
Protection from or tolerance to
- Short circuit
- Overcharge (time)
- Overcharge (voltage)
- Over-discharge
- Voltage reversal
- High temperature
- Low temperature
- Misuse
- Abuse
Power output - Load test
|
Failsafe electronics
Marking
Instructions for use
Safety instructions
Mechanical tests
- Crush tests
- Nail penetration tests
- Shock test
- Vibration test
- Impact test
- Drop test
Environmental tests
- Heating
- Temperature cycling
- Altitude
- Humidity
- Exposure to fire
|
The published safety standards specify the method of testing and the limits with which the product must comply.
DEF Standards
Cells used in military applications usually have to meet more stringent requirements than those used in consumer products.
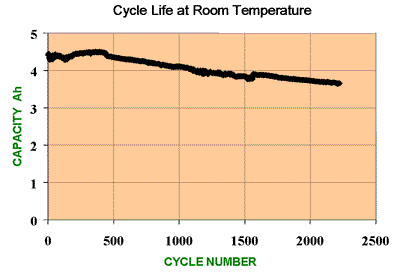
This is perhaps the most important of the qualification tests. Cells are subjected to repeated charge - discharge cycles to verify that the cells meet or exceed the manufacturer's claimed cycle life. Cycle life is usually defined as the number of charge - discharge cycles a battery can perform before its nominal capacity falls below 80% of its initial rated capacity. These tests are needed to verify that the battery performance is in line with the end product reliability and lifetime expectations and will not result in excessive guarantee or warranty claims.
Temperature, charge/discharge rates and the Depth of Discharge each have a major influence on the cycle life of the cells (See the page on
Cycle Life) Depending on the purpose of the tests, the temperature and the DOD should be controlled at an agreed reference level in order to have repeatable results which can be compared with a standard. Alternatively the tests can be used to simulate operating conditions in which the temperature is allowed to rise, or the DOD restricted, to determine how the cycle life will be affected.
Similarly cycle life is affected by over charging and over discharging and it is vital to set the correct voltage and current limits if the manufacturer's specification is to be verified.
Cycle testing is usually carried out banks of cells using multi channel testers which can create different charge and discharge profiles including pulsed inputs and loads. At the same time various cell performance parameters such as temperature, capacity, impedance, power output and discharge time can be monitored and recorded. Typically it takes about 5 hours for a controlled full charge discharge cycle. This means testing to 1000 cycles will take 208 days assuming working 7 days per week 24 hours per day. Thus it takes a long time to verify the effect of any ongoing improvements made to the cells. Because the ageing process is continuous and fairly linear, it is possible to predict the lifetime of a cell from a smaller number of cycles. However to prove it conclusively in order to guarantee a product lifetime would require a large number of cells and a long time. For high power batteries this could be very expensive.
Load testing is used to verify that the battery can deliver its specified power when needed.
The load is usually designed to be representative of the expected conditions in which the battery may be used. It may be a constant load at the C rate or pulsed loads at higher current rates or in the case of automotive batteries, the load may be designed to simulate a typical driving pattern. Low power testing is usually carried out with resistive loads. For very high power testing with variable loads other techniques may be required. A Ward-Leonard controller may be used to provide the variable load profile with the battery power being returned to the mains supply rather than being dissipated in a load.
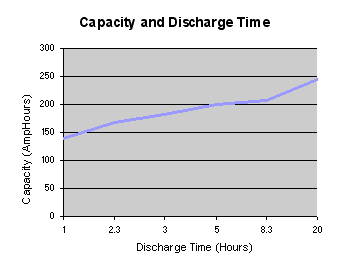
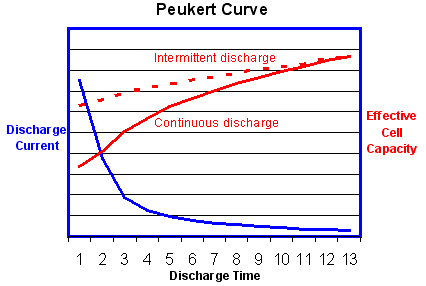
Note that the battery may appear to have a greater capacity when it is discharged intermittently than it may have when it is discharged continuously. This is because the battery is able to recover during the idle periods between heavy intermittent current drains. Thus testing a battery capacity with a continuous high current drain will not necessarily give results which represent the capacity achievable with the actual usage profile.
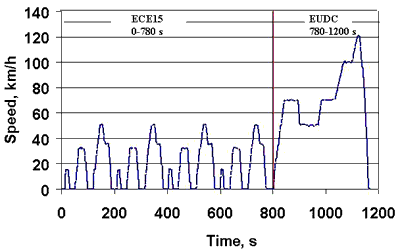
Load testing is often required to be carried out with variable load levels. This may simply be pulsed loads or it could be more complex high power load profiles such as those required for electric vehicle batteries. Standard load profiles such as the Federal Urban Driving Schedule (FUDS) and the Dynamic Stress Test (DST) specified by the United Sates Advanced Battery Consortium (USABC), in the USA, and the United Nations Economic Commission for Europe specification (ECE-15) and the Extra Urban Driving Cycle (EUDC) in Europe have been developed to simulate driving conditions and several manufacturers have incorporated these profiles into their test equipment.
ECE-15 Simulated Driving Cycle
While these standard usage cycles have been developed to provide a basis for comparison, it should be noted that the typical user doesn't necessarily drive according to these cycles and is likely to accelerate at least twice as fast as the allowed for in the standards.
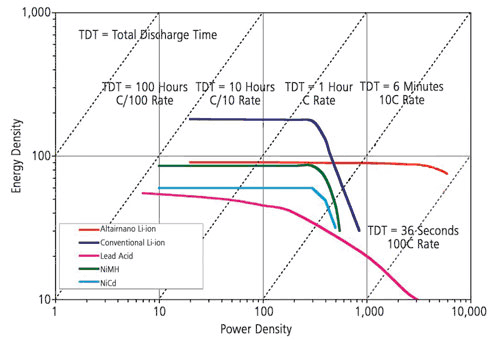
Battery thermal management is critical for high-power battery packs. Obtaining accurate heat generation data from battery modules is essential for designing battery thermal management systems. A calorimeter is used to quantify the total amount of heat generated by the battery while it is cycled through its charge/discharge cycles. This is essentially an insulated box into which the battery is placed which captures and measures the heat generated the battery during cycling. The system is calibrated by comparing the heat generated by the battery with the heat generated by a known heat source.
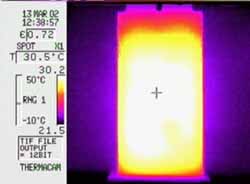
Thermal imaging is used to check for "hot spots" which would indicate points of high thermal stress in the cell or the battery pack. It is a photographic technique which records the intensity of the infra red radiation emitted by a subject using a special camera. The image on the left is of a lithium ion pouch cell after a prolonged discharge at 4C. In this case the temperature is evenly distributed within the cell and the cell terminals are running cool. These tests can help to identify problems such as overheating, inadequate heat sinking or air flow, undersized current conductors and interference from neighbouring cells or devices. The images can also be used to determine the best location for the temperature sensors used in protection circuits.
Electromagnetic Compatibility (EMC) testing
Electromagnetic compatibility (EMC) is the ability of electronic and electrical equipment and systems to operate without adversely affecting other electrical or electronic equipment OR being affected by other sources of interference such as power line transients, radio frequency (RF) signals, digital pulses, electrical machinery, lightning, or other influences.
Note that EMC concerns both the emission of electromagnetic interference (EMI or radio frequency interference RFI) by a product or device and the product's susceptibility to EMI emitted from other sources. The interference may be conducted through power or signal cables or the chassis of the equipment, it may be propagated through inductive or capacitive coupling or it may be radiated through the atmosphere.
Just because batteries are DC devices we can not assume that they are immune from EMC problems. At MPower we have seen the battery protection circuitry in a two way radio disabled by RF interference from the handset's transmitter. Similar problems are possible in automotive applications where the power cabling is notoriously noisy due to interference from the ignition systems and transients from electric motors and switches. While the battery itself may not emit RF interference, the same can not be said of the charger. Many chargers use switch mode regulators which are also notorious for emitting electrical noise. Radiated EMI can be critical to such applications as heart pacemakers, medical instrumentation, communications equipment and military applications.
As with many problems prevention is better than cure and it is wise to start considering EMC at the earliest possible stage of the design to avoid costly design changes when the project is submitted for final approval. This may involve system design choices such as operating frequencies, circuit layouts and enclosure design and the avoidance of designs with high transient currents.
Various techniques are used to minimise the effects EMI. Sensitive parts of the circuit may be physically separated from sources of interference, the equipment may be enclosed in a sealed metal box, individual parts of the circuits may be shielded with metal foil, filters can be added to cables to filter out the noise,
EMC testing involves specialised test equipment and facilities. Testing must be carried out in an environment free from other sources of EMI. This usually means an anechoic chamber or a Faraday cage. Special wide range signal sources and sensitive receivers are needed to generate and measure the interference.
Some examples of EMC requirements are give in the section on
Standards
Process audits
Conducting a process audit of the cell manufacturer's production facilities is further way of gaining confidence in the cells under consideration however this option is usually available only to major purchasers of high volume or high cost cells. Unless you are one of these you will have to rely on your friendly pack maker who possibly qualifies for special treatment.
The process audit involves verifying that the cell maker has appropriate quality systems in place and that these are being fully implemented at every stage of the manufacturing process. To be effective this task needs to be conducted by a team with specialist industry knowledge. Again this is a job best left to your pack maker who should have the necessary experience and credibility with the cell makers.
Inspection and Production Testing
The purpose of inspection production testing is to verify that the cells which have been purchased and the products built with them conform to agreed specifications. These tend to be short tests carried out on 100% of the throughput or on representative samples. The composition of the materials from which the components are made should not be overlooked. We have seen examples of unscrupulous suppliers plating connectors with a gold coloured alloy rather than the gold specified and using cheap plastics which buckle in the heat rather than the high quality plastics required.
Typical tests include both mechanical and electrical tests. The components are checked for dimensional accuracy and sample subassemblies are subject to weld strength testing of the interconnections. Electrical parameters measured include the internal impedance and the output voltage of the cell or battery pack with or without a load. The battery is also submitted to short duration charging and discharging pulses of about 2 milliseconds to check that the unit accepts and can deliver charge.
Battery packs are normally subjected to more comprehensive testing to ensure that the electronics are functioning correctly. The protection circuit is checked by applying a short circuit across the battery terminals for 1 or 2 seconds and checking that the current path is cut within the prescribed period and that the battery recovers afterwards. The output of the fuel gauge is checked and if the battery has built in memory, the data such as cell chemistry code, date and serial number are read out and recorded to permit traceability.
This is normally carried out by the cell manufacturer but in some circumstances it could be the responsibility of the battery pack assembler. In any case the cells must be tested to ensure that they are ready to deliver current.
Performance Monitoring
Performance monitoring is used to verify whether the cell is continuing to perform as required once it is in use in the application for which it was specified. These are individual tests specified by t he user.
There are no simple direct measurements, such as placing a voltmeter across the terminals, to determine the condition of the battery. The voltmeter reading may tell us something about the state of charge (with an enormous margin of error), but it cannot tell us how well the battery will deliver current when demanded.
Internal Resistance
It is necessary to know the internal resistance of the cell in order to calculate the Joule heat generation or I2R power loss in the cell, however a simple measurement with an ohmmeter is not possible because the current generated by the cell itself interferes with the measurement.
To determine the internal resistance, first it is necessary to measure the open circuit voltage of the cell. Then a load should be connected across the cell causing a current to flow. This will reduce the cell voltage due to the IR voltage drop across the cell which corresponds to the cell's internal resistance. The cell voltage should then be measured again when the current is flowing. The resistance is calculated by ohms law from the voltage difference between the two measurements and the current which is flowing through the cell.
Measuring a battery's open circuit voltage is not a reliable measure of its ability to deliver current. As a battery ages, its internal resistance builds up. This will reduce the battery's ability to accept and to hold charge, but the open circuit voltage will still appear normal despite the reduced capacity of the battery. Comparing the actual internal resistance with the resistance of a new battery will provide an indication of any deterioration in battery performance.
State Of Charge (SOC)
For many applications the user needs to know how much energy is left in a battery. The SOC is also a fundamental parameter which must be monitored and controlled in
Battery Management Systems. The methods of estimating the SOC are explained in the section on
State Of Charge.
State Of Health (SOH)
The State of Health is a measure of a battery's ability to deliver the specified current when called upon to do so. It is an important factor for monitoring battery performance once it has entered into use. This is treated briefly in the section below and more fully in the section on
State Of Health.
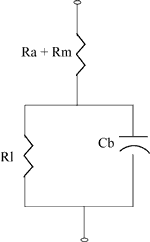
Impedance and Conductance Testing
The discussion about the battery equivalent circuit in the section on
Performance Characteristics shows that we can expect the battery impedance to increase with age.
Battery manufacturers have their own definitions and conventions for Impedance and Conductance based on the test method used. Though not strictly correct they serve their purpose.
The test method involves applying a small AC voltage "E" of known frequency and amplitude across the cell and measuring the in phase AC current "I" that flows in response to it.
The Impedance "Z " is calculated by Ohm's Law to be Z=E/I
The Conductance "C" is similarly calculated as C=I/E (the reciprocal of the impedance)
Note that the impedance increases as the battery deteriorates while the conductance decreases. Thus C correlates directly with the battery's ability to produce current, that is, its capacity, whereas Z gives an inverse correlation. The conductance of the cell therefore provides an indirect approximation to the State of Health of the cell. This measurement can be refined by taking other factors into account. These are outlined in the page about
State of Health.
In addition to impedance and conductance these tests will obviously detect cell defects such as shorts, and open circuits.
These test methods can be used with different cell chemistries however different calibration factors must be built into the test equipment to take into account differences in the aging profiles of the different chemistries.
Impedance and conductance testing are reliable, safe, accurate, fast and they don't affect the battery performance. They can be carried out while the battery is in use or they can be used to continuously monitor the battery performance, avoiding the need for load testing or discharge testing.
Note that DC measurements do not recognise capacitance changes and therefore measurements of the internal resistance of the cell do not correlate so well with the SOH of the cell.
Using a conventional ohmmeter for measuring the resistance of the cables, contacts and inter-cell links is not satisfactory because the resistance is very low and the resistance of the instrument leads and the contacts causes significant errors. More accuracy can be achieved by using a Kelvin Bridge which separates the voltage measuring leads from the current source leads and thus avoids the error caused by the volt drop along the current source leads. See also charger
voltage sensing.
Battery Analysers
Battery analysers are designed to provide an quick indication of the
State of Health (SOH) of the battery. Some analysers also have the dual function of reconditioning the battery.
There are no industry standards for this equipment, mainly because there is no standard definition of State of Health. Each equipment manufacturer has their own favourite way defining and measuring it, from a simple conductance measurement to a weighted average of several measured parameters and the test equipment is designed to provide the corresponding answer. This should not be a problem if the same equipment is used consistently, however it does cause problems if equipment from different manufacturers is used to carry out the tests.
Failure Analysis
Cell failure analysis is best carried out by the cell manufacturers. Only they will have the detailed specifications of the cell mechanical and chemical components and it normally requires access to expensive analytical equipment such as electron microscopes and mass spectrometers which they should be expected to have. More information see
Why Batteries Fail and
Lithium Battery Failures